SCHOLASTIC APTITUDE TEST 1999
CHEMISTRY
| Time: 1 Hour |
Max.Marks : 60
|
Instructions:
(1) Answer must be written either in
English or the medium of instruction of the candidate in high
school.
(2) There will be no negative marking
(3) Use of calculators or graph papers
is not permitted
(4) There are SIX Questions. Answer all
the questions.
I. Multiple choice. Each
question has only one correct answer.
Indicate the correct answer by A,B,C,D. (5x1 = 5 Marks)
- The electronic configuration
of elements A,B,C and D are (2,8,1) (2,8,2) (2,8,6) and (2,8,7)
respectively which of them can make an ion with two
negative charges.
- A neutral atom of
an element has a nucleus with a nuclear charge 13 times
and mass 27 times that of hydrogen nucleus. How many
electrons would be in its stable positively charged ion.
- At any moment
before reversible reaction attains equilibrium it is
found that
| A) The
velocity of the forward reaction is increasing and
backward reaction is decreasing |
| B) The
velocity of backward increasing and forward
decreasing |
| C) The
velocities of both forward and backward reaction are
increasing |
| D)
Velocities of both forward and backward reaction
decreasing |
- When the same
amount of Zn is treated separately with excess of
sulphuric acid and excess of sodium hydroxide the ratio
of volumes of hydrogen evolved is
| A) 1:1 |
B) 1:2 |
C) 2:1 |
D) 9:4 |
- Which of the four
conditions density of the nitrogen gas will be largest
| A) STP |
B) 273 K and
2 atm |
| C) 546 K and
1 atm |
D) 546 K and
2 atm |
II. Write the balanced Chemical
Equations for following chemical changes:
(5x1=5
Marks)
- Zn granules are dropped in to
very dilute solution of nitric acid.
- Mixture containing CO2
and CO is passed over the redhot coke.
- Ammonium nitrate is strongly
heated.
- White phosphorus is treated
with caustic soda solution.
- KI is treated with con.H2SO4
solution.
III. Explain the following
Facts: (10x2 = 20 Marks)
(Write the chemical equations
wherever necessary)
- NO2 is reddish
brown gas but on cooling it becomes colourless but on
heating again it gains the colour.
- Potassium chromate in water
forms yellow solution. On addition of acid this solution
turns orange, on addition of NaOH to the orange solution
again becomes yellow.
- Calcination is carried in the
absence of air whereas roasting is carried in the
presence of air.
- Gold is insoluble in either
in con. HCl (or) HNO3 separately while it is
soluble in aqua-regia a mixture of both.
- Ist Ionisation energy of Nitrogen is greater
than oxygen where as reverse the case for 2nd
Ionisation value.
- To protect the Iron from
corrosion it is covered with zinc (Galvanisation) even
though zinc is more reactive than Iron.
- When Nitric acid is exposed
to light slowly turns yellow.
- In the detection of
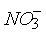 through Brown
ring test, concentration H2SO4 slowly
added through the walls of Test tube.
through Brown
ring test, concentration H2SO4 slowly
added through the walls of Test tube.
- Ammonium chloride is a salt,
but its aq.solution terns blue litmus red.
- Metallic zinc displaces
Hydrogen gas from dilute H2SO4 but
metallic copper doesnot.
IV. Fill in the blanks (5x1
= 5 Marks)
- Ratio of weights of O2
and O3 is 1:1 so the ratio of their moles is
___________________.
- 250 ml of 6M HCl, 650 ml of 3M
HCl were mixed together. The volume of water should be
added to make the solution 3M is _____________________ ml.
- If a hospital buys 8 grms of
Co60, the amount of same cobalt left after 20
years would be (t1/2 of Co60 = 5
years) _________________.
- The central atom of a
molecule of the type AX3 has one lone pair in
its valance shell. The shape of the molecule is
_______________.
- An element which can exist as
(+) ve ion in acidic solution but also as a negative ion
in basic solution is said to be ______________________.
V. Differentiate the following:
(10x2 =20 Marks)
- oils - fats
- ionisation - electrolysis
- thermoplastics -
thermosetting plastics
- soap - detergent
- sublimation -
liquification
- metallic conductor -
electrolytic conductor
- hygroscopic solids -
deliquescent solids
- cathode - anode
- bond energy - binding
energy
- ionic product -
solubility product
VI. Solve the following: (2x2½=5
Marks)
- Sodium bicarbonate (baking
soda), NaHCO3, can be purified by dissolving
it in hot water (600C), filtering to remove
insoluble impurities, cooling to 00C to
precipitate solid NaHCO3, and then filtering
to remove the solid, leaving soluble impurities in
solution. Any NaHCO3 that remains in solution
is not recovered. The solubility of NaHCO3 in
hot water at 600C is 164 g/L. Its solubility
in cold water at 00C is 69 g/L. What is the
percent yield of NaHCO3 when it is purified by
this method?
- Hash Chemical Company sells a
test kit for determining the concentration of chloride
ion in domestic water supplies. The kit contains a silver
nitrate, AgNO3, solution that is added drop by
drop to a 23.0 mL water sample to which an indicator has
been added. When sufficient silver nitrate has been added
to convert the chloride ion completely to silver chloride,
AgCl, the solid produced turns orange. The concentration
of the silver nitrate solution is such that each drop
used to reach the color change corresponds to 12.5 mg of
Cl- per litre of water tested.
(a) What mass of chloride ion
(mg/L) is contained in a water sample that requires 12 drops
of test solution to reach the color change?
(b) What is the molar
concentration of Cl- in the sample tested in part(a)?
(c) If the sample size used is
5.75 mL instead of 23.0 mL, to what chloride ion
concentration (mg/L) does 1 drop of the test solution
correspond?
(d) If 20 drops of the silver
nitrate test solution equals 1.00 mL, what is its molar
concentration of AgNO3?