SCHOLATIC APTITUDE TEST 1996
CHEMISTRY
| Time: 1 Hour |
Max.Marks
: 60
|
Instructions:
(1) Answer must be written either in
English or the medium of instruction of the candidate in high
school.
(2) There will be no negative marking
(3) There are THREE Sections. Answer all
questions of a section at one place
(4) Use of calculators or graph papers
is not permitted
SECTION-I
Fill in the Blanks (20 x 1 = 20
Marks)
1. The actual species exist in the aqueous
solution of H2SO4 are
____________________________
2. He, Ne, Ar, Kr, Xe are called inert
gases, but to create inert conditions during the chemical
reactions nitrogen gas is used. The inert behaviour of
nitrogen is due to ____________________
3. pH of the aqueous solution increases
with dilution. pH of the solution increases by one unit on 10
fold dilution. One litre of solution of pH=6 is diluted to
100 litres pH of the resulted solution is nearly
_______________
4. Four quantum numbers of the electron is
useful in determining the spatial position of the electron in
given atom. The four quantum numbers of the differentiating
electron in scandium atom (Sc=21) is ______________
5. Radio activity is the property of
instable nucleus. Atoms with instable nucleus emits a , b , g radiations.
The instability of the nucleus is due to __________________
6. Bond dissociation energy of the Cl2
is greater than that of F2 as well as Br2.This
is due to ________
7. Zinc decolourises the blue colour of
aqueous blue vitriol, but silver does not do so. This is
because ___________________
8. Every atom and ion contain three
fundamental particles. The difference between the mercury (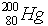 ) atom and
mercurous ion is ______________ (in terms of number of
fundamental particles)
) atom and
mercurous ion is ______________ (in terms of number of
fundamental particles)
9. Chemical formula of the compound which
is commonly used to detect presence of Cl- ion in
drinking water is _____________________ and this compound can
be detected by using ______________ aqueous solution
10. Most of the elements exist in the
nature only in the form of compounds this is due to
___________
11. __________________________ is used to
detect the presence of water.
12. When a compressed gas at a temperature
is allowed to expand through a small porous plug or small
orifice the temperature of gas ________________________
13. Self addition to form macro molecules
is characteristic reaction exhibited by many unsaturated
compounds. PVC obtained by ______________________
14. During chemical bonding BF3
molecule always accepts pair of electrons to form a bond.
This is due to __________________
15. Allotropy is the phenomenon of an
element in which the element shows different physical
properties and similar chemical properties. This is due to
_______________________________
16. Be, Mg, Ca, Sr and Ba metals are called
alkaline earth metals. This is due to _________________
17. A covalent bond is formed by the equal
sharing of electrons from the bonded atoms. The actual forces
responsible for holding the atoms together in the molecule
are _______________________
18. According to collision theory rate of a
reaction is directly proportional to the number of effective
collisions per second. The meaning of the effective
collisions is __________________________
19. _________________________ is used as a
flux in blast furnace to remove _________________ in the
extraction of iron
20. Theoretically the orbitals present in
hydrogen atom are __________________________
SECTION-II
| Explain the following: |
(10 x 21/2
= 25 Marks)
|
1. Some chemical reactions are reversible
and some are irreversible.
2. The boiling points of hydrogen compounds
of oxygen family can be represented graphically as follows:
3. Solubility is generally defined as the
number of grams of the solute present in 100 gm of the
solvent at a given temperature. Generally as the temperature
increases solubility increases but in few cases it decreases
with increase of temperature.
4. Molality of the solution is independent
of temperature where as molarity is dependent on temperature.
5. According to the equation 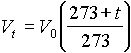 ,
when t = -2730C Vt becomes zero. i.e. a
gas would occupy no volume at all
,
when t = -2730C Vt becomes zero. i.e. a
gas would occupy no volume at all
6. What type of graphs are obtained for P
vs V under isothermal conditions of T1, T2,
and T3 where T3 > T2 >
T1. To compare draw them in a single figure.
7. Only under certain conditions real gases
obey the Boyle’s law.
8. Generally rate of the chemical reaction
exponentially decreases with time.
9. pH of the 0.1 M HCl is 1, but 0.1 M
acetic acid solution is greater than 1.
10. Glass is called as supercooled liquid.
SECTION-III
| Solve the Problems: |
(15 Marks)
|
1. 6.3 gm of oxalic acid crystals (C2O4H2.2H2O)
in 250 ml flask is treated with excess of concentrated
sulphuric acid. Heat the flask gently. The two gas components
which are released passed through the caustic soda. Finally
one gas is collected over water.
a) what is the function of concentrated H2SO4?
b) what are the gas components ?
c) what gas component is absorbed by
caustic soda?
d) if the absorption is chemical process,
write the chemical equation
e) how many grams of each component is
released?
f) what is the pressure of the gas
collected over water, if the volume of the gas is 2 litres
and temperature of gas is 270C (aqueous tension =
30 mm Hg at 270C). ( 6 )
2. When salt `A’ is treated with
concentrated H2SO4 , `B’ (solid)
and `C’ (gas) are formed. `C’ forms white fumes
with NH3 . `B’ is treated with coke and lime
stone and heated to 8000C, a solid `D’ is
formed. `D’ is treated with water to obtain a solution E
and a solid F. When excess CO2 gas is passed
through this solution precipitate `G’ is formed. `G’
on strong heating gives `E’. Write the sequence of
chemical reactions and identify the substances from A to G. (
3 )
3. 0.1525 gm of an unknown liquid on
evaporation displaces 35.05 ml of air and collected in a
graduated glass burette. The pressure of displaced air is 730
mm Hg and the temperature is 200C. Calculate the
molecular weight of the liquid. ( 3 )
4. In the chemical reaction 2X+3Y  4Z
4Z
1.0 mole of X,
1.25 mole of Y and 2.5 mole of Z are found at equilibrium in
a 1.0 litre reaction mixture.
a) calculate the value for K
b) calculate the value for K if the
same mixtrue had been found in a 2.0 litre vessel.
( 3 )
![]() ) atom and
mercurous ion is ______________ (in terms of number of
fundamental particles)
) atom and
mercurous ion is ______________ (in terms of number of
fundamental particles)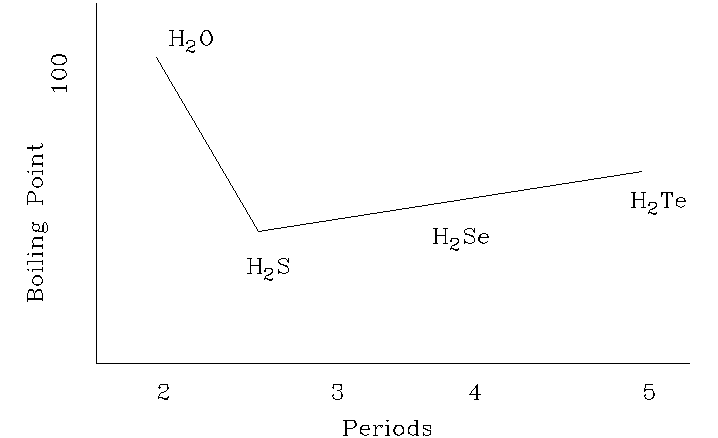
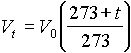 ,
when t = -2730C Vt becomes zero. i.e. a
gas would occupy no volume at all
,
when t = -2730C Vt becomes zero. i.e. a
gas would occupy no volume at all