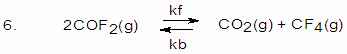|
CHEMISTRY
Instructions: (1) Answer must be written either in English or the medium of instruction of the candidate in high school.
1. The value of K (equilibrium constant, rate
constant, solubility constant) changes with temperature. But
radioactive decay constant is independent of temperature.
1. An equivalent in acid-base reaction and an equivalent in oxidation–reduction reaction
1. A fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 gm carbon dioxide, 0.69 gm of water and no other products. A volume of 10 lit (measured at STP) of this gas is found to weigh 11.6 gm. (a) Calculate 2. At a given temperature, the degree of ionization (fraction dissociated) of water is found to be 1.8x10-9. Calculate the ionization constant and the ionic product of water at this temperature. 3. Suppose the gas-phase isomerisation reactions reach equilibrium at a fixed temperature. Express the equilibrium mole fractions of A,B and C in terms of equilibrium constants, K1, K2 and K3. 4. A sample of P2O5 contains some impurity. 0.405 gm sample is reacted with water and the resulting solution requires 42.5 ml of 0.25 M NaOH solution. The salt resulted is monobasic acid salt. Calculate the percent impurity. 5. What will be the resultant pH when 200 ml of an aqueous solution of HCl (pH=2) is mixed with 300 ml of an aqueous solution of NaOH (pH=12).
K=2 for the reaction at 10000C. If a 5L mixture contains 0.105 mole COF2, 0.22 mol CO2 and 0.055 mol CF4 at 10000C. (a) Will the mixture be at equilibrium? 7. For the reaction (i) A B ; Kc = 2 For the reaction A D what is the Kc? 8. Four elements coded A,B,C and D form a series of substances e.g. AB, B2, CB3, DB2 and DB3. If the atomic number of these elements are not necessarily in the order are 13, 19, 26 and 35. Write down extra nuclear electronic structures of these elements. From this information and the formula of the compounds, allocate A,B,C and D with appropriate atomic numbers. Mention the nature of bonding in each of them. 9. Calculate the molecular weight of hydrogen fluoride if density of this gas is 3.17 g/L at 300K and 1.0 atm pressure. What information can you deduce from the result? 10. Anhydrous copper sulphate turns to blue
from colourless on expose to atomosphere and again make it colourless
by some substance (X). When copper sulphate is added to water a blue
colour solution is resulted with some turbidity. The turbidity
disappears by the addition of a drop of HCl solution. On placing Iron
rod in it, a greenish solution is resulted. A chocolate like
precipitate is resulted when a drop of potassium ferrocyanide solution
is added to the blue colour solution of copper sulphate. Black
precipitate is resulted by passing H2S gas through copper sulphate solution but the precipitate is soluble in conc.HNO3. |